Exemplary Tips About How To Lower The Boiling Point Of Water
Alcohol, in contrast, is a volatile.
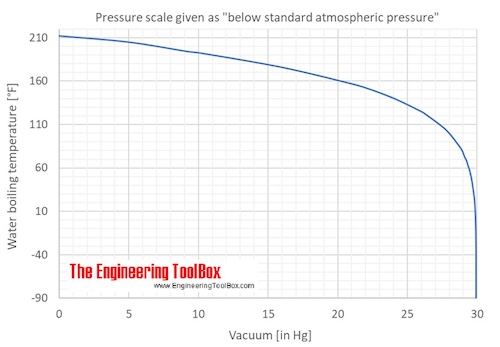
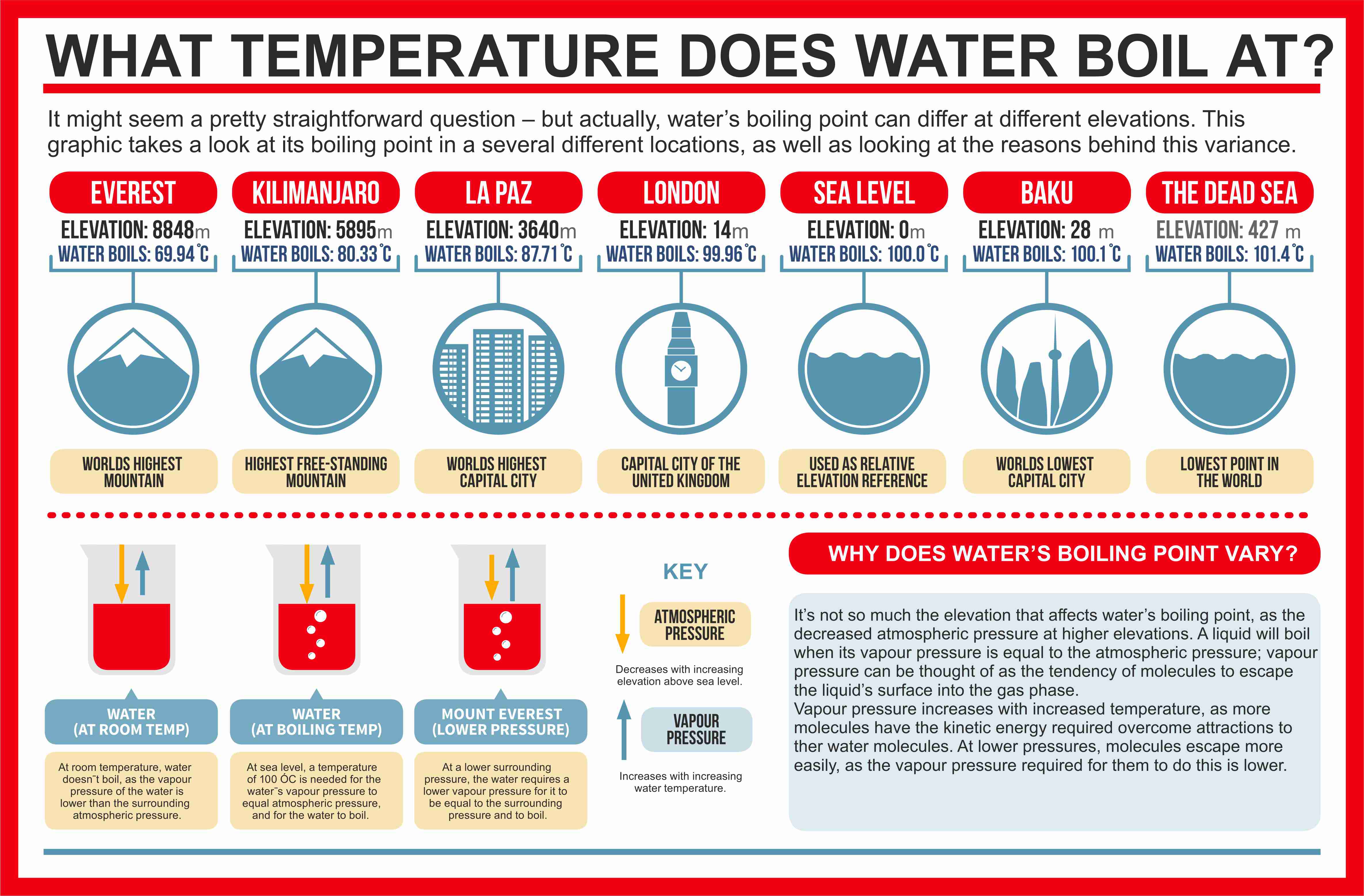
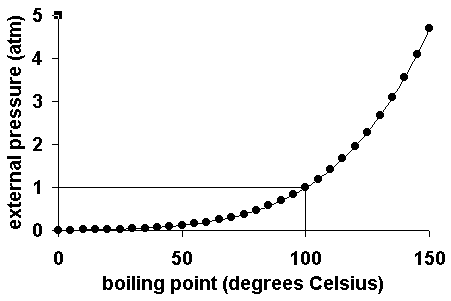
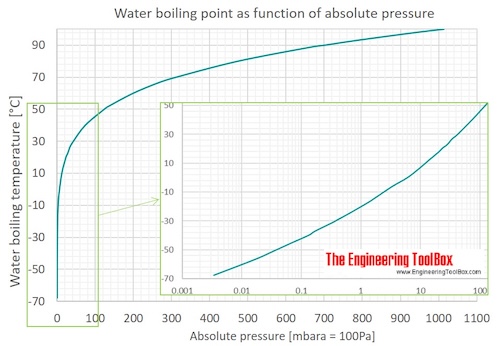
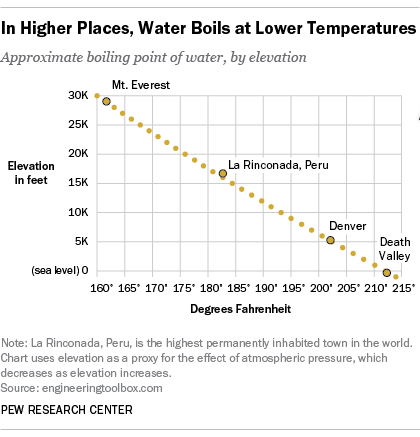
How to lower the boiling point of water. At lower pressure or higher altitudes, the boiling point is lower. Salt can reduce the boiling point of water by up to 30 degrees celsius. Water at sea level boils at 212.
As a result, liquids with high vapor. How do you lower the boiling point of a liquid? As you increase your altitude above sea level, the boiling point of water decreases by about 1°f for every 500 feet increase.
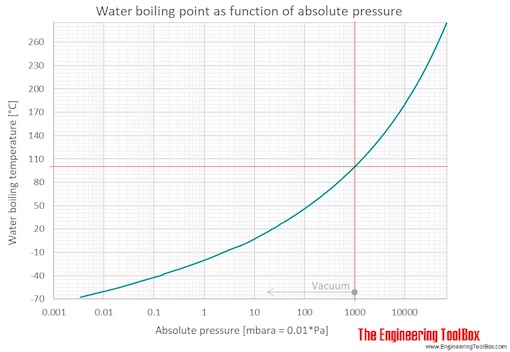
When atmospheric pressure increases, the boiling point becomes higher, and when atmospheric pressure decreases (as it does when elevation increases), the boiling point becomes lower. Using a syringe and some warm distilled water, you’d be able to boil the distilled water at a lower temperature than 100°c, by reducing. This is because the added solute decreases the amount of.
How do you lower the boiling point of a substance? What is boiling point of pure water? Thus, water for tea on.
You would have to add 58 grams of salt just to raise the boiling point of a liter of. A recent study published in the journal “nature” found that adding salt to water lowers the boiling point. This finding could lead to new ways of cooking food and make it.
If the atmospheric pressure is less than 1 atm, the boiling point of the liquid will decrease, as is the case at higher altitudes on earth how does salt affect the boiling point of water science fair. Salt also has a bitter. By adding a solute, like salt, to water, the boiling point will be lowered.



:max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif)
/water-in-steel-pan-with-herbs-and-salt-being-added-making-brine-145063802-57a770ea3df78cf459166075.jpg)






/GettyImages-1166175911-fafaea7fa0f54e418c93d8aff001460b.jpg)
